Angular Probability Densities
Sunday, March 6, 2022
What are the Angular Probability Densities?
The angular parts of the probability density function from the wave function is
Looking at the and angular probability density functions, we see that all are cylindrically symmetric, and the wave function is spherically symmetric:
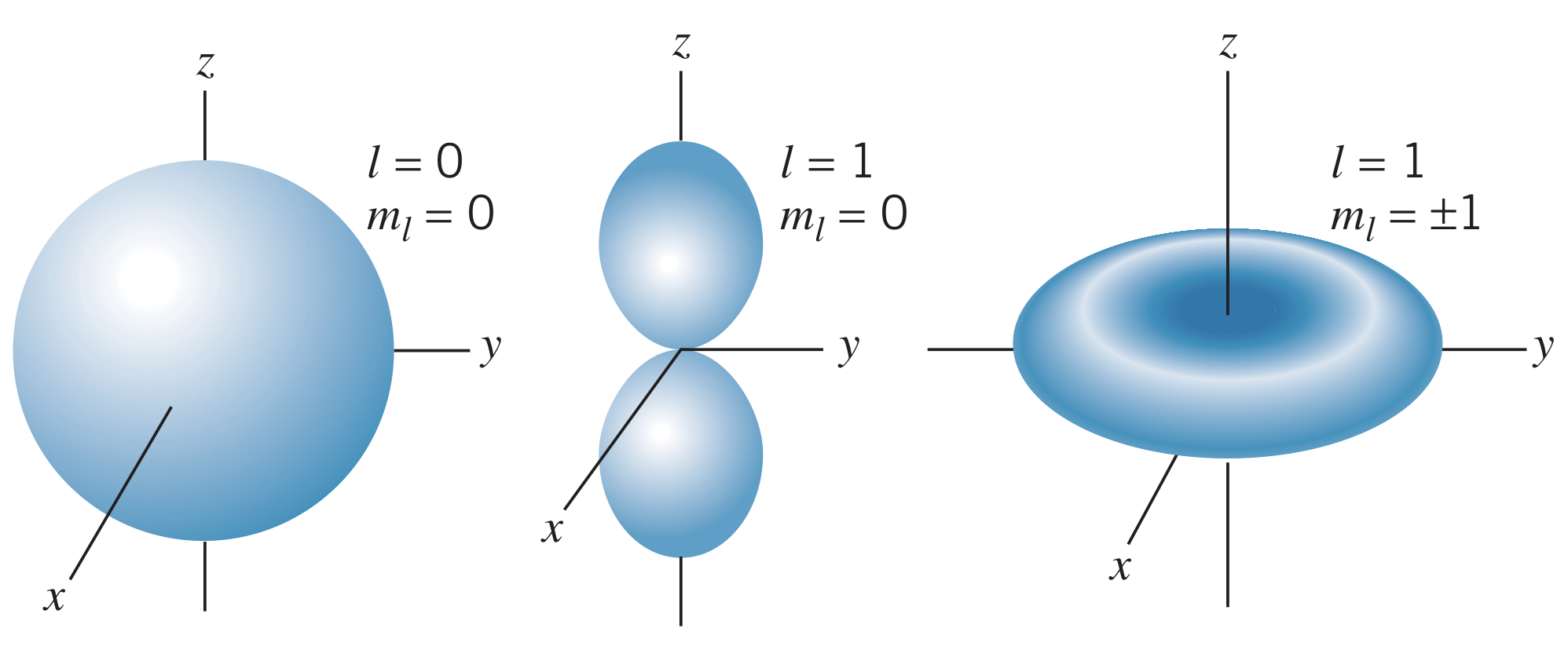
For the wave functions, when , the density function forms two regions along the -axis. This makes sense given the fact that the direction of the angular momentum vector is in the -plane. Additionally, for the wave functions, the maximum projection of the angular momentum vector is along the -axis, making the electrons spend most of their time near the -plane.