Electronic States in Multielectron Atoms
Monday, March 14, 2022
Atomic Subshells
Looking at the energies for electron orbitals show a pattern where the orbital has the least energy, followed by the and orbitals whose energies are relatively close to one another. This pattern continues with higher energy levels, where different orbitals are almost grouped together with the same energies.
Penetrating Orbits
Two of the orbitals ( and ) penetrate the inner orbits (meaning they have a large probability density for small values of ) significantly. The orbital however has negligible penetration, and so has a much higher energy than the other two orbitals.
The same phenomenon occurs with the orbitals, where the and subshells penetrate so much that their energies almost coincide with the orbital.
Although penetrating orbits spend more time closer to the nucleus than non-penetrating ones, they also spend more time farther away, averaging out to approximately the same average radius.
Atomic Shells
The set of orbitals with a certain value of is called an atomic shell. They are designated by letter, as shown below:
| Shell |
Levels with certain values of and are called subshells, such as or . The maximum number of electrons allowed in each of these levels is . Below is a table with some atomic subshell capacities:
| Subshell | Capacity | ||
|---|---|---|---|
Note: these tables only represent the "outer" or valence electrons since they describe how electrons fill the shells. The properties of electrons change as the of an atom changes For instance the nineteenth electron of potassium () is very different from the nineteenth electron of lead (). Instead, we describe the inner electrons by shells, such as the shell.
The Periodic Table
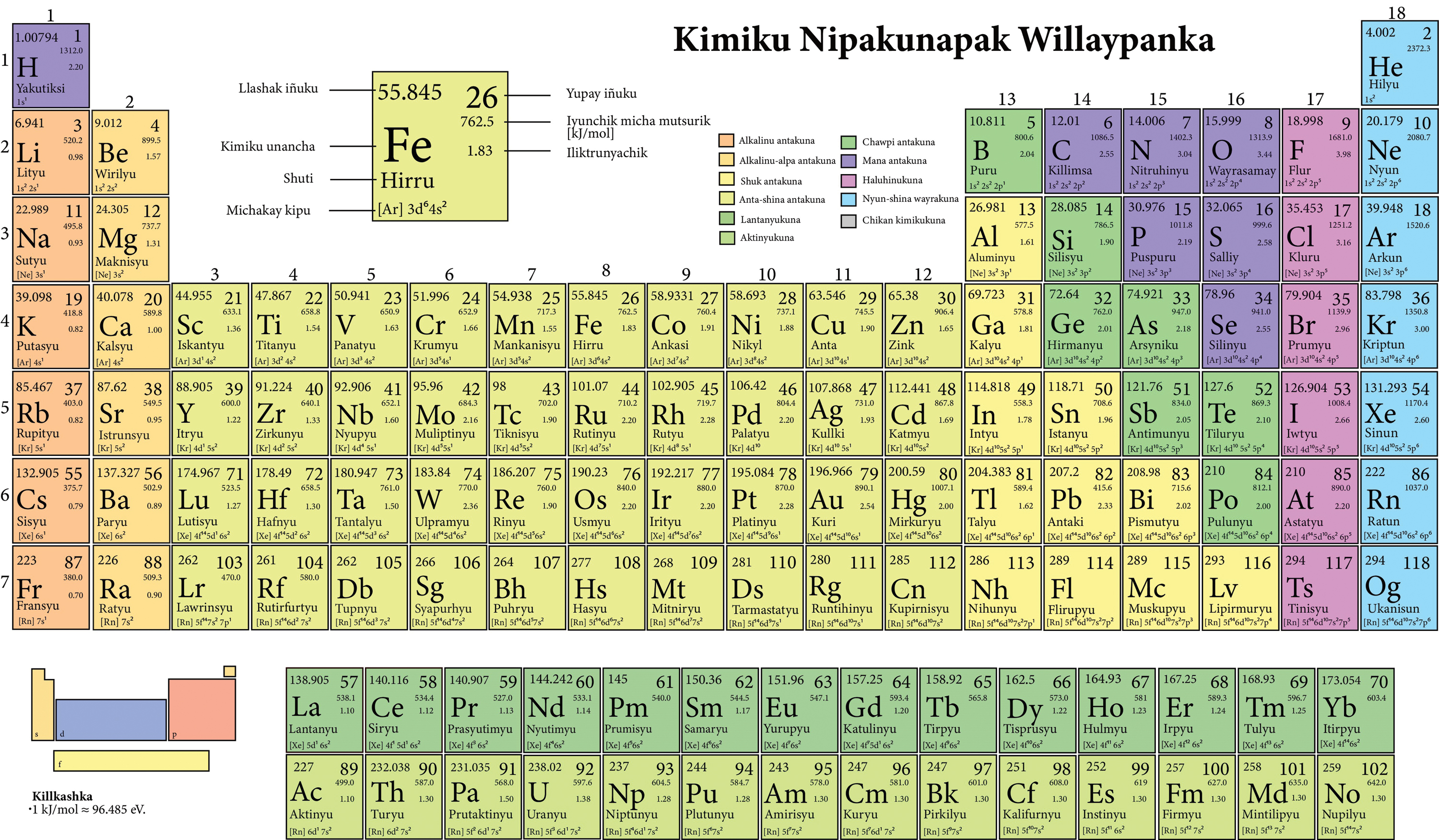
The periodic table arranges all the elements by increasing atomic number () such that the vertical columns (groups) contain elements with similar properties.
Electron Configurations
We have seen that to fill electronic subshells, there are two rules:
- The capacity of each subshell is , called the Pauli exclusion principle
- The electrons must occupy the lowest energy states available
Electron configurations are written for each element using a notation to identify the subshell and number of electrons it contains. For instance, hydrogen has an electron configuration of , since it has one electron in the suborbital.
In general, orbitals are filled in order, however there are some discrepancies such as copper (), which would be expected to fill the suborbital before the suborbital. However, an electron completing the orbital requires slightly less energy, so copper's electron configuration is .