Line Spectra
Sunday, February 6, 2022
Types of Radiation
Atoms can either emit electromagnetic radiation in continuous spectra or as discrete wavelengths of light. An example of a continuous spectrum would be thermal radiation by a hot object. On the other hand, atoms such as neon excited in a glass tube emit certain wavelengths of light and do not emit any others.
Absorption Spectra
When white light (with all wavelengths of light) is passed through a gas, some wavelengths of light will be absorbed, leaving a dark line in the rest of the rainbow spectrum of the light. This is called the sample's absorption spectrum, and is not always the same as its emission spectrum.
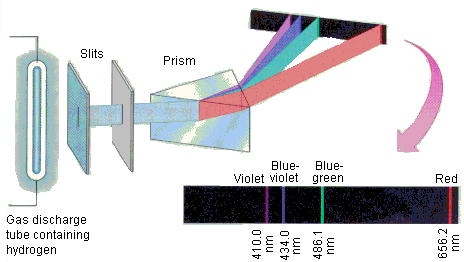
Hydrogen's Spectra
Johann Balmer noticed that hydrogen's emission lines follow the following formula quite closely:
Where . This is known as the Balmer formula, and the series of emission lines that follow it are known as the Balmer series.
All of the groupings of lines for hydrogen fit the following formula:
Where and is the wavelength of the series limit.
Ritz combination principle
Converting hydrogen's emission wavelengths to frequencies show that certain pairs of frequencies add to give other frequencies found in the spectrum.